
(PDF) Rituximab Treatment and Longterm of Patients With Autoimmune Encephalitis Real
New research presented this week at ACR Convergence, the American College of Rheumatology's annual meeting, shows immunocompromised patients using rituximab (a drug used to treat diseases like.

LongTerm Remissions of Severe Pemphigus After Rituximab Therapy Are Associated with Prolonged
Patients with autoimmune inflammatory rheumatic disease (AIIRD), such as rheumatoid arthritis (RA) and systemic lupus erythematosus, are at higher risk for serious infections, which is likely due to both disease-related immune dysfunction and immunosuppressive medication use. Because of this risk, vaccination is an important part of care.

(PDF) Low rate of longlasting remissions after successful treatment of immune thrombocytopenic
Figure 1 (A) A potential therapeutic approach to immune compromised B cell-depleted patients with COVID-19 on/after Rituximab therapy. Mild to moderate disease. MoAbs, monoclonal antibodies; CP, convalescent plasma; Ig, Immunoglobulins. (B) A potential therapeutic approach to immunocompromised B cell-depleted patients with COVID-19 on/after Rituximab therapy.
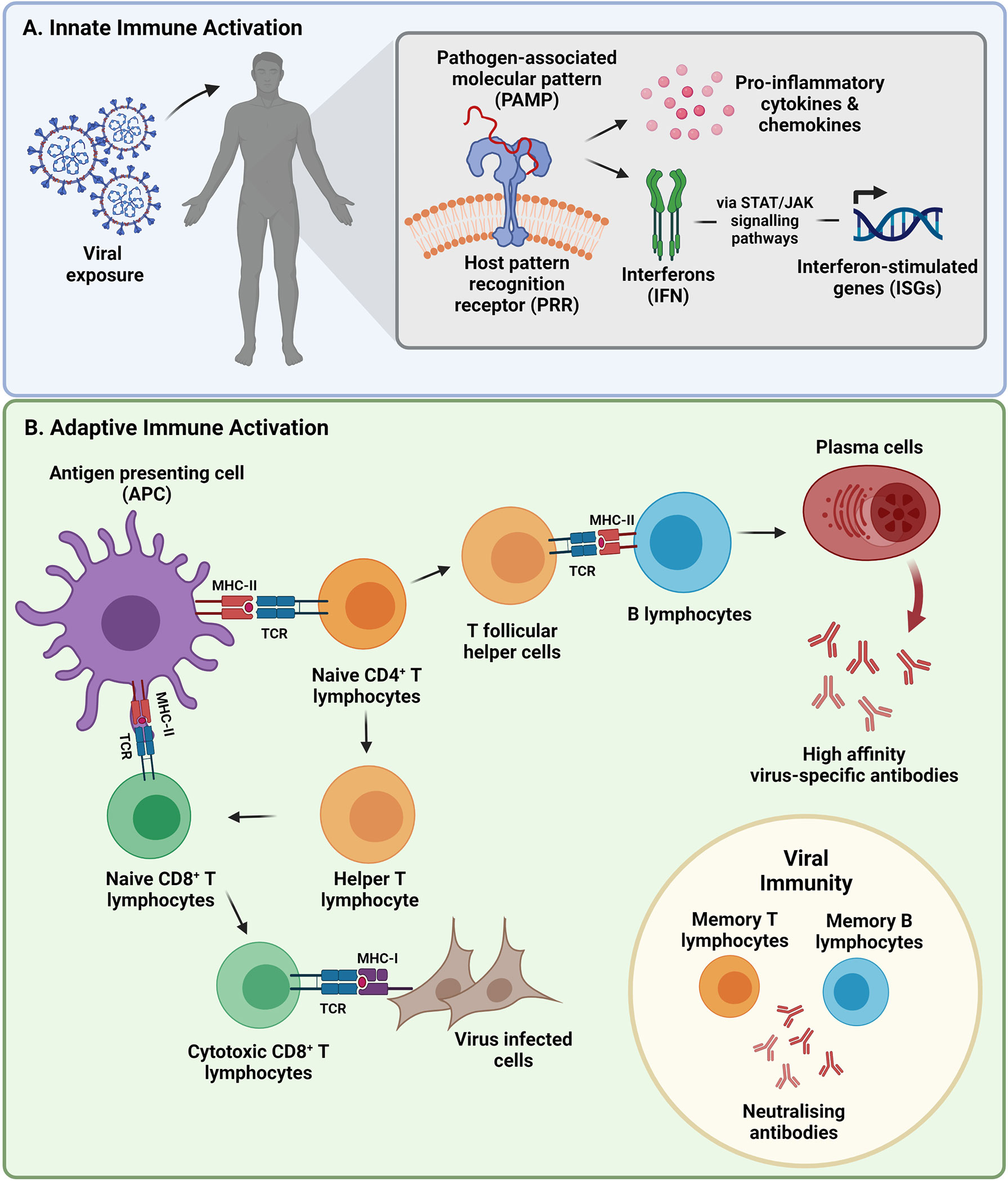
Basic immunology abbas chapter 12 ameladock
Statement 2c: In patients with immune-mediated diseases treated with rituximab who require optimal vaccine immunogenicity, we recommend that immunization be deferred to ⩾5 months after the last dose and at least 4 weeks prior to the subsequent dose of rituximab. Strong recommendation; low-level evidence. Live attenuated vaccines: Herpes zoster
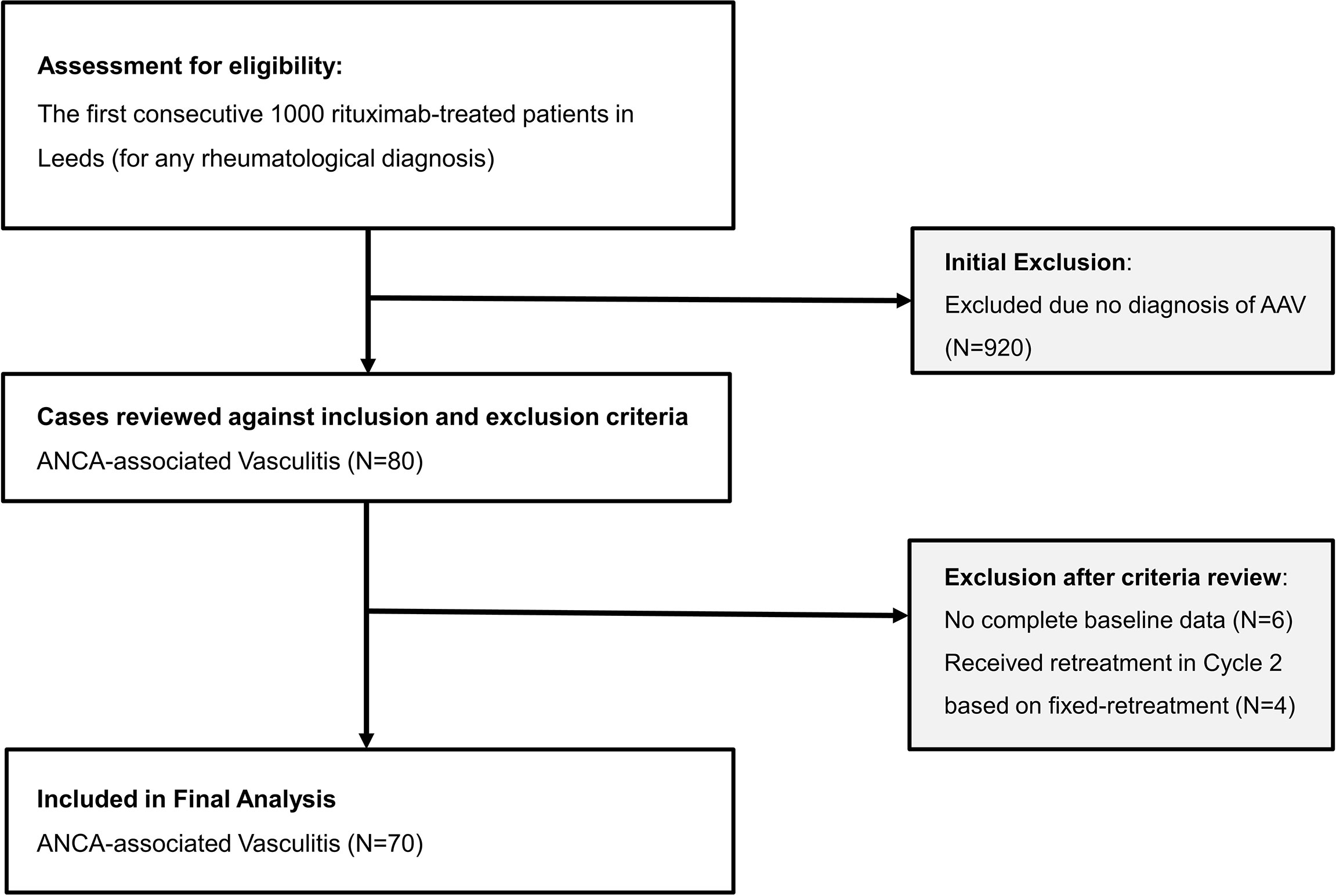
Frontiers A Personalized Rituximab Retreatment Approach Based on Clinical and BCell
Fifteen of the 126 patients had been fully vaccinated against COVID-19 prior to starting rituximab treatment, most just a few weeks before. Ten of these patients generated a blocking antibody response to the virus. In six of them, that response persisted at least four months after they started rituximab treatment.
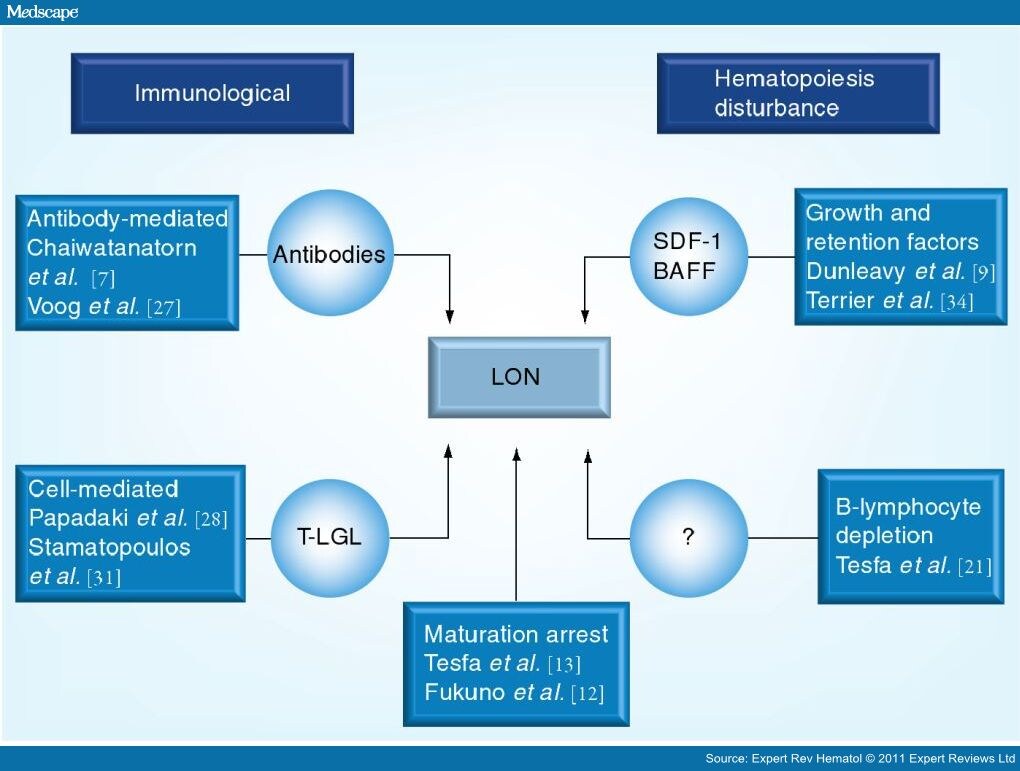
Lateonset Neutropenia Following Rituximab Therapy Page 4
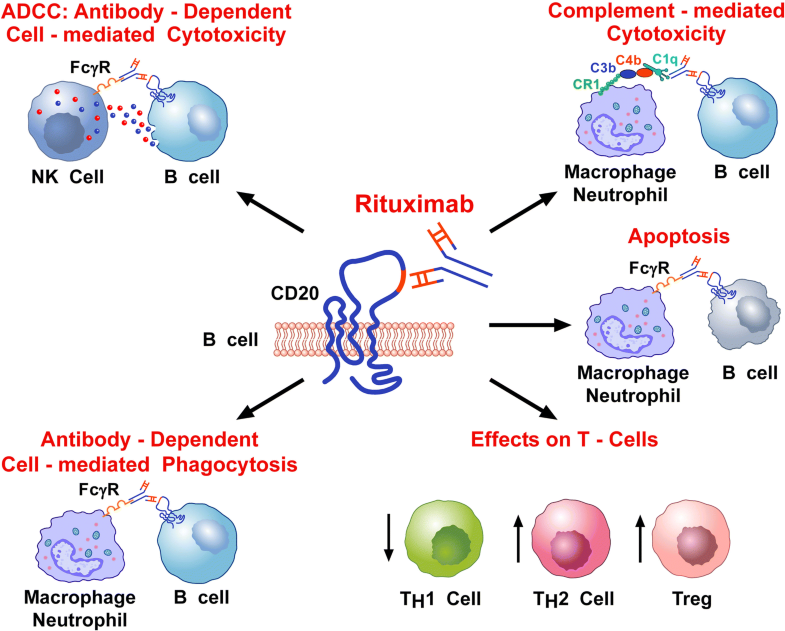
Rituximab is a chimeric monoclonal antibody that binds to the CD20 surface marker expressed on B cells. This includes precursor B cells (pre-B cells) and mature and memory B cells. Following antibody binding, B cells die by a number of mechanisms including antibody-dependent cell-mediated cytotoxicity, complement-dependent cytotoxicity, and.

Mean relative changes in CD20+ cell counts after infusion of rituximab... Download Scientific
Beginning with therapy and continued 6 mo after therapy or until normalization of ALC (≥1.2 k/uL) (e.g. Maintenance Anti-CD20 , rituximab, obinutuzumab) No routine prophylaxis No routine prophylaxis No routine prophylaxis Acyclovir 400 mg BID Hepatitis B screen prior to initiation Throughout all chemotherapy cycles
:max_bytes(150000):strip_icc()/3232654_color1_edited-5c362536c9e77c0001885f8b.png)
Autoimmune Disease Types, Symptoms, And More 9E1
This section describes situations in which vaccines are recommended outside of the routine-age-based recommendation because the risk for vaccine-preventable disease is increased due to altered immunocompetence. Persons with altered immunocompetence generally are recommended to receive polysaccharide-based vaccines (PCV13, PPSV23, and Hib), on.

Complete disappearance of pulmonary rheumatoid nodules after long term rituximab treatment case
The Panel recommends following the CDC's COVID-19 vaccination guidance for people who are moderately or severely immunocompromised. This guidance includes information on the use of the updated 2023-2024 mRNA vaccines, which target the SARS-CoV-2 Omicron variant lineage XBB.1.5.

Effects and safety of rituximab in systemic sclerosis an analysis from the European Scleroderma
Intravenous immune globulin ("IVIG") is a product made up of human antibodies that can be given intravenously (through a vein). Antibodies are proteins your body makes to help you fight certain infections. Each antibody made by your body is slightly different because it fits like a lock and key to every foreign substance (such as a piece of a.
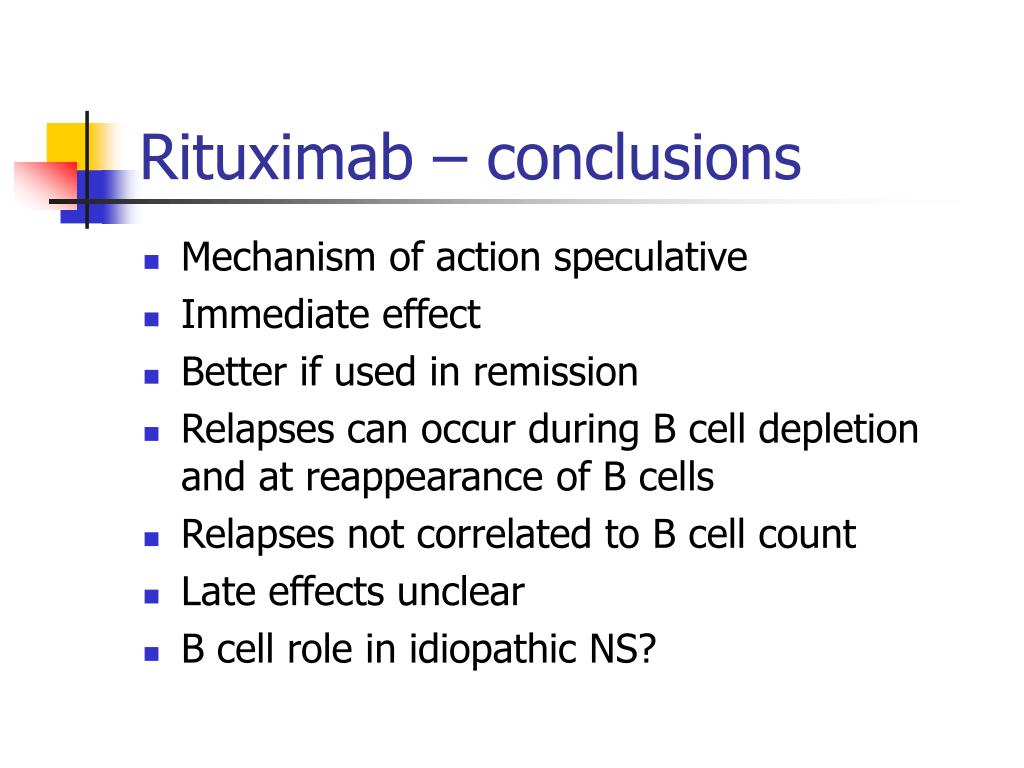
PPT Rituximab Treatment in Nephrotic Syndrome PowerPoint Presentation ID497439
One article reported long-term infectiousness among 3 patients, 1 with untreated HIV, 1 with a heart transplant, and another with rituximab-treated rheumatoid arthritis. "If you look at the clinical presentations, they're quite diverse," Gupta said.

(PDF) Single in usion of rituximab for persistent steroiddependent minimalchange nephrotic
• In the case of long-acting rituximab maintenance regimens, delaying intervals between rituximab infusions could be considered for patients where the risk of disease flare is deemed low and the risk of 3 adverse outcomes with COVID-19 infection is high.. If you are immunocompromised, protect yourself from COVID-19, https://www.cdc.gov.
Immunosuppressive therapy with rituximab in common variable immunodeficiency SpringerLink
Intact humoral and innate immunity appear necessary to eradicate SARS-CoV-2 in some individuals being treated with rituximab. The American Society of Hematology reports on 17 B-cell depleted patients with prolonged COVID-19 who were treated with convalescent plasma. All but 1 patient had symptom improvement within 48 hours.
Rituximab maintenance therapy a step forward in follicular lymphoma Haematologica
Of note is that among 303 drugs examined in the study, only one, rituximab, a monoclonal antibody preparation that targets antibody-producing B cells, was found to be associated with a substantially increased risk of death compared with medically similar hospitalized COVID-19 patients. Researchers analyzed data on 153 cancer patients taking.

Is rituximab maintenance effective longterm for follicular lymphoma after an autologous stem
Once treatment with rituximab has been completed, recovery of immune cell function can take 3-12 months, and even longer in some cases. It may be used as a once-only dose or as a long term therapy. Due to the effects on lymphocytes, patients on rituximab therapy are considered immune suppressed and are at a greater risk of vaccine preventable.
(PDF) Common variable immunodeficiency unmasked by treatment of immune thrombocytopenic purpura
Accumulating data suggest that treatment with anti-CD20 therapy, such as rituximab and ocrelizumab, puts patients at considerably increased risk of developing severe outcomes from COVID-19 (risk ratios ranging from 1·7 to 5·5 have been reported).1,2 This reported risk emphasises how important it is that these patients develop protective immunity via COVID-19 vaccinations, but, unfortunately.
- Hard Rock Cafe Max Euweplein Amsterdam
- Zeven Zussen Deel 4 En 5
- 1 Euro To Krone Norwegian
- Jaguar F Pace Reviews 2023
- Goed Betaalde Banen Voor Vrouwen Zonder Diploma
- Rode Urine Bieten Hoe Lang
- Mag Je Werken Met Corona 2023
- True Heart Emote Last Seen
- In From The Cold Season 2
- How Much Does A Wardrobe Stylist Cost